RESEARCH ACTIVITIES
Research in my group focuses on synthetic inorganic chemistry and on material manufacturing. Here we want to address important challenges in the fields of solar energy, catalysis and photochemistry. In addition, through our chemical explorations, we hope to gain fundamental insight into the nature of bonding and reactivity of group 14 compounds. Within my group we pursue the bottom-up approach, which means that we first synthesize main group compounds and subsequently use these derivatives as precursors for our materials. Consequently, researchers in the Haas group will be exposed to a number of advanced synthesis and characterization techniques, including material characterization. The two main research directions are depicted in Chart I.
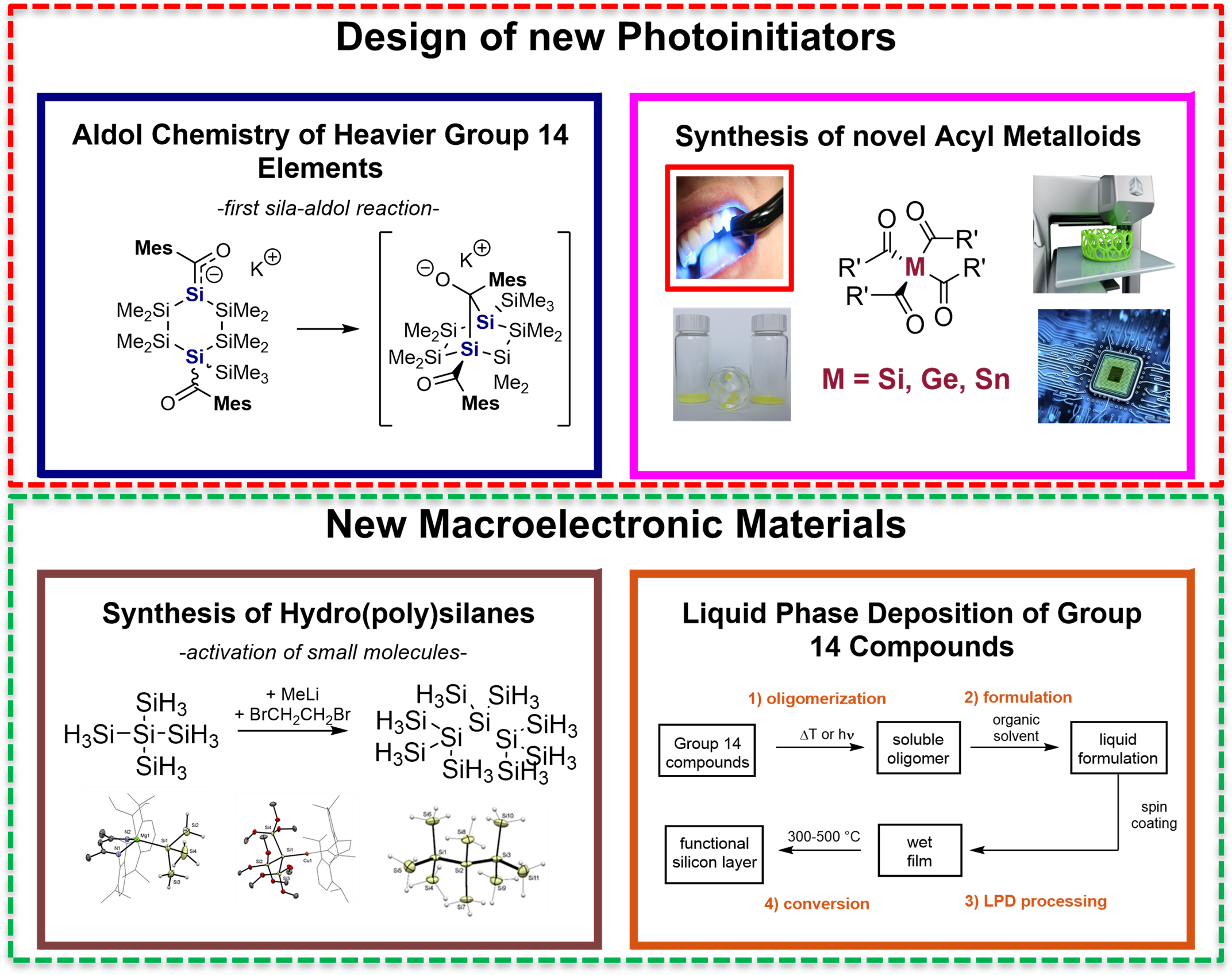
Design of New Photoinitiators
The first research line is the synthesis of new high performance non-toxic photoinitiators. In the last decades the demand and application of high performance photochemically produced polymers has been immensely growing. Nowadays, their use is no longer restricted to the manufacture of microelectronic devices, coatings, adhesives, inks, printing plates, optical waveguides but, also enters fields of medicine (dental filling materials, artificial tissue, heart valves etc.) and fabrication of 3D objects. In the world of photopolymerization, high demands on the performance of the products exist. Moreover, they have to be produced by sustainable, economic and environmentally friendly procedures. To meet the strict qualifications, especially for medical applications, new types of non-toxic photoinitiating systems are necessary.
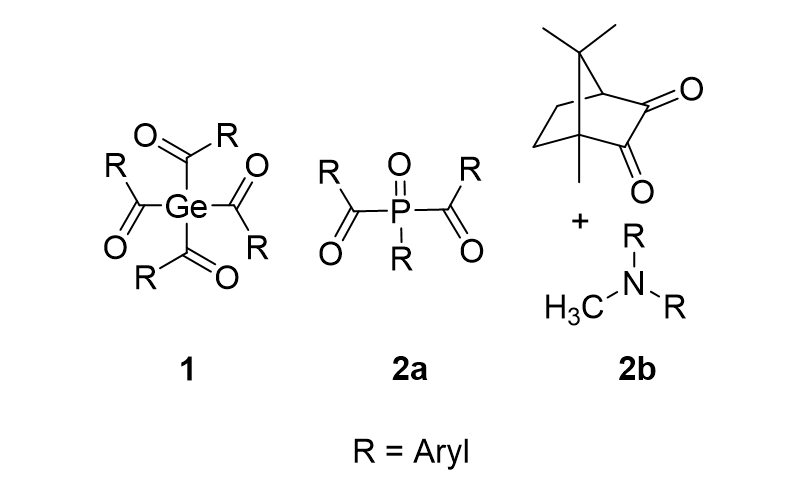
Among the promising PI systems, acylgermanes (Figure 1 compound class 1) can act as suitable radical precursors generating acyl- and germyl-centered radicals upon irradiation, which add very rapidly to double bonds of various monomers. Moreover, they offer the advantages of significantly red-shifted absorption bands and reduced toxicity compared to the frequently applied phosphorus-based (Figure 1 compound class 2a) and campherchinon/amine (Figure 1 compound class 2b) systems.
Within the last years, we have been active in the field of photo-induced radical reactions and the synthesis of group 14-based photoinitiators for radical polymerizations. Our contributions comprise synthetic, photophysical, photochemical and material manufacturing aspects. This is well documented in several peer-reviewed publications in high-impact journals.
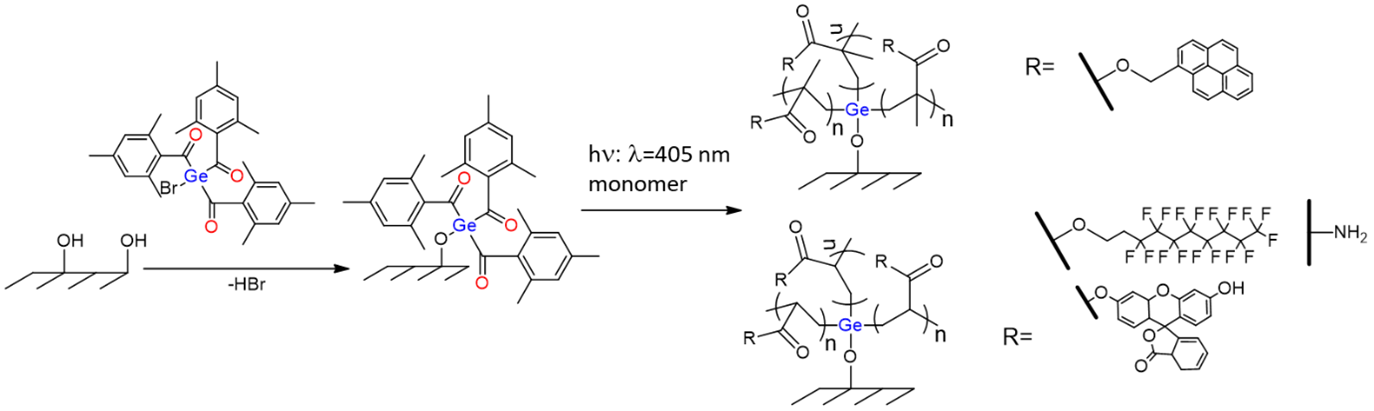
A representative example of the bottom-up approach of my group is the recently submitted work, which deals with immobilized novel germanium-based photoinitiators. Here we could show that the immobilized Ge-based photoinitiator is capable of efficiently initiating a visible light-induced and surface-mediated polymerization reaction of various functional monomers (Scheme 1). Considering the advantages of germanium-based photoinitiators, this may open up new perspectives for surface modifications, especially in the field of biomedicine.
This research topic in particular is very collaborative and builds on good contacts in physical, analytical and materials chemistry. Here my appointment can certainly be an asset to the research environment at the Graz University of Technology. I think that several groups at different institutes of the Graz University of Technology are predestined for possible collaboration on this topic. At the chemical department I think that Prof. Trimmel can be an ideal partner to characterize our obtained material. At the physical department Prof. Gescheidt is an expert in the broad field of light and photo physics, here I think that a cooperation can result in publications with high impact.
Furthermore, we have currently three industrial cooperation partners (Ivoclar Vivadent AG and Evonik Creavis GmbH, Altana AG) funding this research line. More information about these projects is permitted by these partners.
We also interested in very fundamental questions related to this topic. Here we want to utilize the long-overlooked aldol reaction of the higher homologous of carbon as alternative and mild bond formation methodology. The reason for our interest is based on the fact, that the classical aldol reaction, is one of the most important biosynthetic tools for life on Earth. The versatility and selectivity associated with this process are among the most extensively studied of all synthetic methods. For other group 14 elements, however, such bond-forming reactions were unknown until our group discovered the first aldol reaction of heavier carbon homologues (HCH aldol reaction) identified in cyclic silanes (see Figure 2).
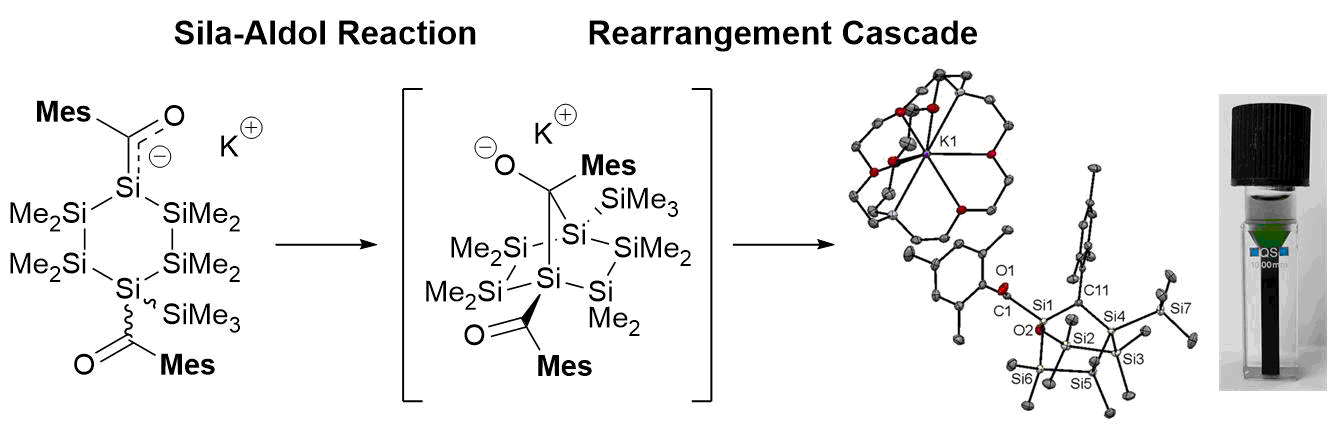
From a synthetic standpoint, extension of the aldol reaction to acylsilanes, acylgermanes, and acylstannanes initiates a fundamental and direct approach towards carbon-group 14 metal based compounds and complements standard techniques such as Wurtz reaction, hydrometalation and transition-metal-catalyzed coupling reactions. Beyond its utility of creating β-hydroxy acyl subunits, the HCH aldol reaction can selectively deliver complex, unnatural frameworks with high non-carbon content that are challenging to access via other methods. During this project we could show that this methodology delivers intra- as well as inter- molecular aldol products. On the basis of the good progress of the project (more than 15 peer reviewed papers in three years). These fundamental questions are the topic of an ongoing FWF-Project.
New Macromolecular Materials
Chemical vapor deposition (CVD) is the state-of-the-art process for making polycrystalline Si films in microelectronic industry. As an alternative, solution processing of silicon-based devices has attracted considerable attention owing to the possibility of low-cost fabrication by printing processes. Moreover, it opens the possibility for large area depositions and patterning materials. Recent studies in our laboratories and by others have demonstrated the principal feasibility of the liquid phase deposition (LPD) and processing of silicon films of satisfactory quality (see Figure 3).
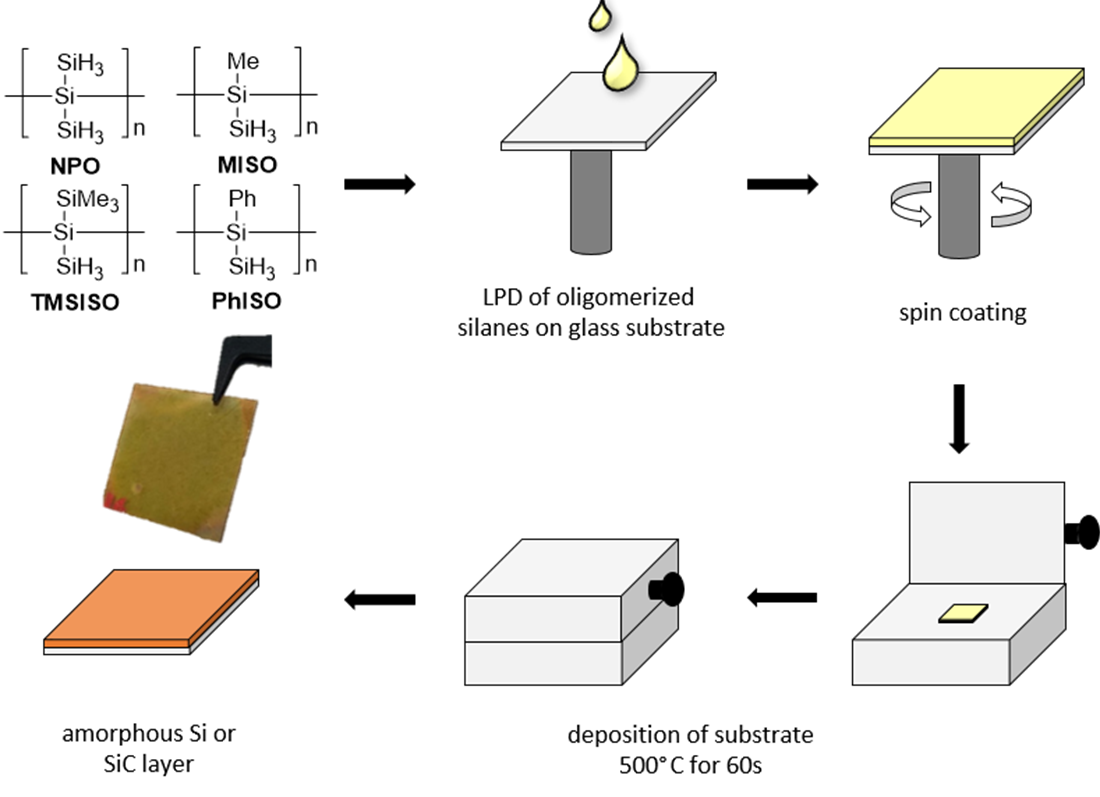
In this context the deposition of silicon-heteroelement thin layer structures is of great interest. Therefore, we want to use hydrosilane precursors for the deposition of functional silicon films, which contain one or more heteroatoms covalently linked to silicon (single source precursors). Our group is the leading research group for the synthesis of hydropolysilanes. Furthermore, we were granted a public funded project on this topic. Again, we follow the bottom-up approach. The first targets were the isolation of single source precursors and a subsequent material manufacturing. A representative example is the just submitted work by our group that demonstrated the usage of single source precursors for the fabrication of carbon doped amorphous films.
In our study, 2-methyl-2-silyltrisilane (3), 1,1,1-trimethyl-2,2-disilyltrisilane (4), 2-phenyl-2-silyltrisilane (5) as well as 1,1,2,2,4,4,5,5-octamethyl-3,3,6,6-tetrasilylcyclohexasilinane (6) were utilized as precursor materials and compared with the parent compound 1 (See Scheme 2).
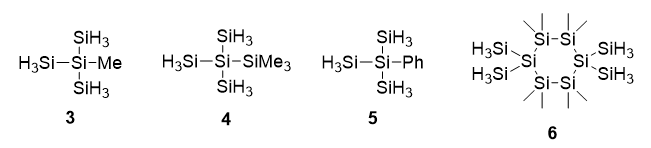
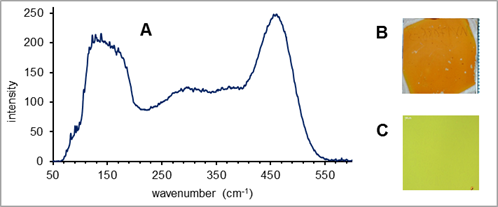
Compounds 3-6 were successfully oligomerized at λ = 365 nm in the presence of catalytic amount of neopentasilane oligomer (NPO). These oligomeric mixtures were used for the preparation of thin layer materials. All thin layer materials were characterized via UV/VIS spectroscopy, light microscopy, spectroscopic ellipsometry, SEM and SEM/EDX. Our results demonstrate that the carbon content and more-over the bandgap can be easily tuned by the usage of these single source precursors (see Figure 4).
Again, this research topic is also very collaborative and builds on good contacts with analytical and material chemistry. Several groups at different institutes of the TU Graz are predestined for possible collaboration on this topic. At the chemical department I think that Prof. Trimmel and Dr. Rath are ideal partner in the field of semiconductor physics. Moreover, Prof. Kothleitner can analyze the obtained films with STM, TEM, Raman or SEM. Furthermore, we were just granted a Christian Doppler Laboratory with Air Liquide as the industrial cooperation partner on this topic. This project aims to provide a deeper understanding of the outcome of chemical transformations involving higher silicon hydrides and to create a synthetic library of previously unknown functional hydrosilanes. Although the project just started, we already have some decisive breakthroughs and will publish this work in a high-impact publication.
DEPARTMENT OF INORGANIC CHEMISTRY | Technical University Graz

